srne stock news fda approval
SRNE gained over 5 in extended trading session on Tuesday after the company FDA cleared its Investigational New Drug application for early stage. 2162022 SRNE entered 45 million bridge load agreement.

Why An Fda Approval Caused Sorrento Therapeutics Pain Nasdaq
Biotech stocks with key binary eventscatalysts - FDA ApprovalPDUFA dates Advisory Committee and Phase 2 3 trial data releases dates are noted.

. Sorrento Therapeutics NASDAQ. 22 2022 GLOBE NEWSWIRE -- Sorrento Therapeutics Inc. For every action there is an equal but opposite reaction.
FDA Approval Highlights High-Risk Case for Sorrento Therapeutics Stock With multiple ways to win SRNE stock looks attractive here July 22 2021 By InvestorPlace Research Staff Sep 23 2020 755. Sorrento Therapeutics SRNE Granted FDA Okay to Proceed With Phase 1 Study Of Intranasal STI-9199 Article Related Press Releases 1. SRNE stock news and headlines to help you in your trading and investing decisions.
SRNE stock price news charts stock research profile. SRNE Stock Heads Up On FDA IND Approval. Use our tools on your road to profit in the stock market.
Biotech Stock Catalyst and FDA Calendar for your biotech stock investing. The SRNE Q42021 10K states page F-8 that in 2021 SRNE loss before tax is 462528000 and SRNE loss per share is 145. SRNE stock price surges on Pfizer Approval.
If that was the deal the FDA made to protect the governments. Current Report Filing 8-k Edgar US Regulatory - 332022 51749 PM Sorrento granted FDA nod to start clinical trial for intranasal COVID-19 therapy Seeking Alpha - 322022 120247 PM. Sorrento Therapeutics SRNE Announces COVISHIELD STI-9167 Antibody Strongly Neutralizes BA2 Omicron Sublineage Virus as Well as BA1 and BA1R346k Omicron Viruses.
Sorrento Announces FDA Authorization To. Scilex Holding Scilex an over 99 owned subsidiary of Sorrento Therapeutics NASDAQ. The company said that the conjugate takes advantage of several technology platforms under development.
For reference SRNE stock 3112022 close price is 218. Based On Fundamental Analysis. SRNE Business Wire - 1312022 51300 PM.
SRNE Sorrento today announced that additional preclinical results demonstrate broad spectrum COVISHIELD STI-9167 neutralizing activity against Omicron BA1 Omicron BA1R346K and the increasingly prevalent sublineage Omicron BA2. Phase 1 catalysts for small-cap companies only are. Ad Our Strong Buys Double the SP.
Sorrento Announces COVISHIELD STI-9199 Antibody Nasal Drops. STI-6129 is a CD38-targeting antibody drug conjugate. Sorrento Therapeutics SRNE Receives a Buy from Dawson James TipRanks - 372022 43030 AM.
SRNE Sorrento today announced that it has received clearance from the FDA for its investigational new drug application IND for intranasal IN STI-9199 COVISHIELD to study the safety and pharmacokinetics in healthy volunteers. SRNE Sorrento has received a supplemental new drug application sNDA approval from the FDA for ZTlido to make efficacy labeling change with clinical data. This but the FDA in quite an unfavorable light Looks like they are playing games with the lives of the American people.
22 rows SAN DIEGO Feb. Get the latest Sorrento Therapeutics Inc. SAN DIEGO March 02 2022 GLOBE NEWSWIRE -- Sorrento Therapeutics Inc.
Dulan Lokuwithana SA News Editor 5 Comments. In the press release Sorrento Therapeutics said that it received clearance from the FDA for its IND for STI-6129. Corporate News FDA.
Amended Statement of Ownership sc 13ga Edgar US Regulatory - 272022 44216 PM Current Report Filing 8-k Edgar US Regulatory - 212022 40535 PM Newman Ferrara LLP Announces Corporate Governance Investigation of Sorrento Therapeutics Inc. Latest SRNE News 030222 1137 AM Sorrento Announces COVISHIELD STI-9199 Antibody Nasal Drops Prevent Productive SARS-CoV-2 Infections When Given 24 Hours Prior to Virus Exposure 030222 1124 AM Sorrento Announces FDA Authorization to Proceed With Phase 1 Study Of Intranasal STI-9199 COVISHIELD a Potent Neutralization Antibody. SRNE Sorrento announced that the company has received FDA clearance to proceed with a global Phase 2 clinical study of resiniferatoxin RTX entitled A Multicenter Phase 2 Study to Assess the Safety and Efficacy of Epidural Resiniferatoxin for the Treatment of Intractable Pain Associated with Advanced Cancer.
Sorrento rises 7 as STI-1558 neutralizes. December 2021 December 14 2021 - Coronavirus COVID-19 Update. Sorrento granted FDA nod to start clinical trial for intranasal COVID-19 therapy Mar.
2021 is expected to be a huge year for penny stocks. RTTNews - Shares of Sorrento Therapeutics Inc. Sorrento Therapeutics - We apply cutting-edge science to create innovative therapies that will improve the lives of those who suffer from cancer intractable pain and COVID-19.
02 2022 1202 PM ET Sorrento Therapeutics Inc. If its possible that there are people out there with more information than us retail investors then its possible they believe our Covid Pipeline was being held back until big pharmas vaccines gained full approval. Once the country realizes that the FDA has been stone walliing SRNEs devives meds the boomerang effect will be sometbing.
For more information contact FDAs Office of Media Affairs at 301-796-4540.

Sizing Up Sorrento Therapeutics Again Nasdaq Srne Seeking Alpha

Sorrento Inks Deal To Buy Acea Therapeutics Shares Gain 3 Nasdaq

With Progress On Multiple Fronts Sorrento Stock Is A Great Buy Analyst Nasdaq

Where Will Sorrento Therapeutics Be In 1 Year The Motley Fool
Sorrento Stock Price Soars 27 As Covid 19 Antibody Trial Gets Green Light

Srne Institutional Ownership Sorrento Therapeutics Inc Nasdaq Stock
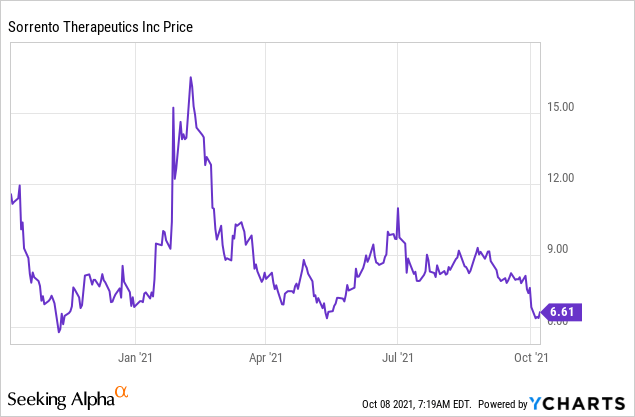
Sorrento Therapeutics Wide Oncology Pipeline And Watch Out For Covid Updates Seeking Alpha
Investors Sorrento Therapeutics
Sorrento Therapeutics Inc Srne Stock Message Board Investorshub